.png)
The UK has approved a fifth Covid-19 vaccine for use in adults, the Medicines and Healthcare products Regulatory Agency confirmed today, February 3.
Nuvaxovid – a two-dose vaccine developed by Novavax – has been found in clinical trials to be around 90% effective against Covid-19, putting it in line with other vaccines currently in use in the UK.
The vaccine, which is similar to flu jabs, is the first 'protein-based vaccine' against Covid-19 to be approved for use in the UK.
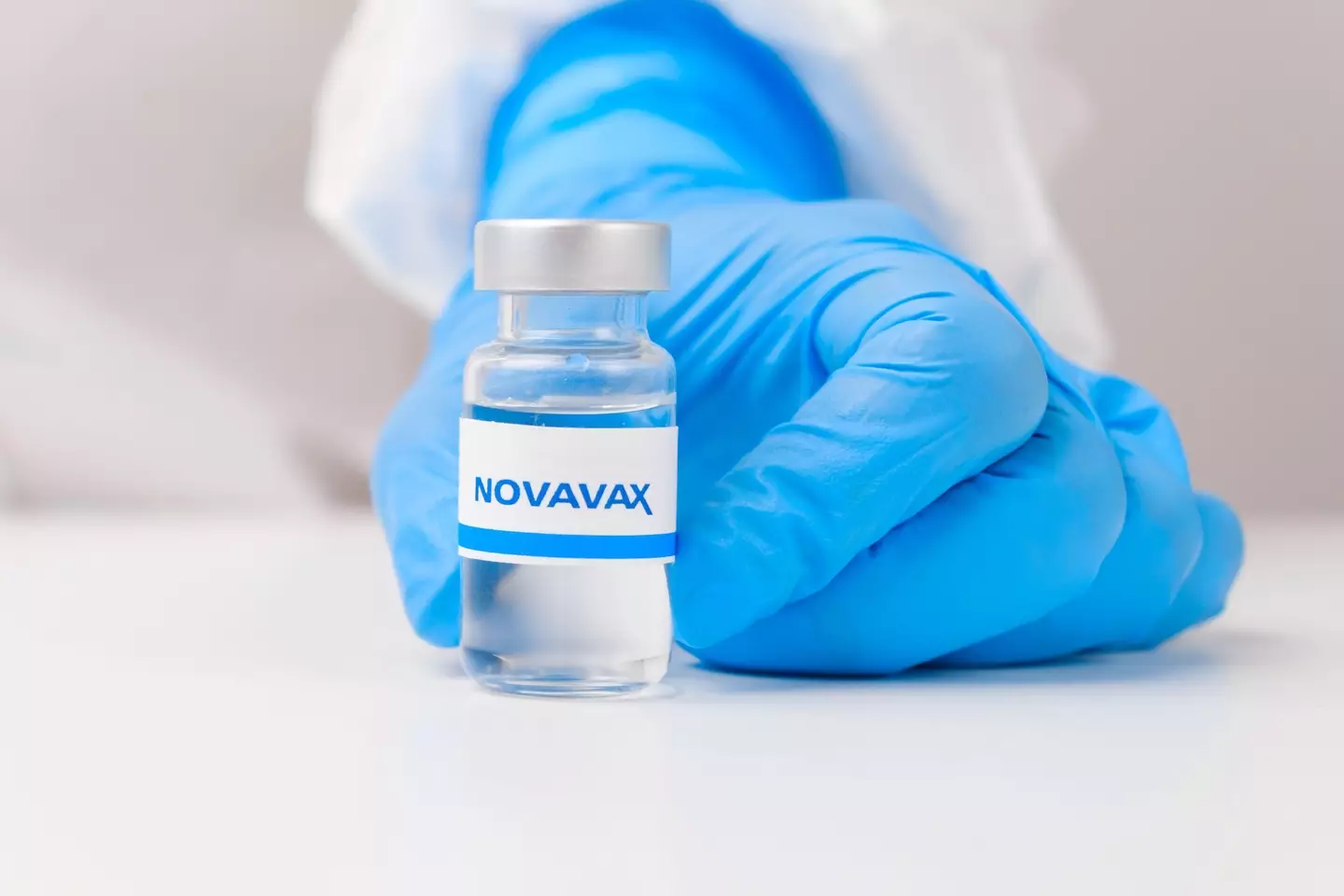
Recent studies have suggested Nuvaxovid may hold up better against the Omicron variant, and may also come with fewer side effects, The Guardian reports.
Advert
The UK had previously agreed a deal with Novavax for 60 million doses of its vaccine, and also collaborated with the country on late-stage clinical trials during its development.
The jab must still be considered by the Joint Committee on Vaccination and Immunisation before it begins being rolled out as part of the country's ongoing vaccination program.
'We are continuing our vital safety work in monitoring the use of all COVID-19 vaccines, to ensure that their benefits in protecting people against COVID-19 disease continue to outweigh any risks,' MHRA Chief Executive June Raine said, per Reuters.
In a statement confirming the decision, Health Secretary Sajid Javid said, 'It is great to see our world-renowned medicines regulator approve another Covid-19 vaccine.
Advert
'I want the UK to be the best place in the world to conduct clinical trials. It’s a testament to the country’s first-rate research and development capabilities for vaccines – with tens of thousands of people taking part in clinical trials here in the UK, contributing to the invaluable research that shows our vaccines are safe and effective.'
It's understood that health agencies hope that the use of more established vaccine technology to develop this jab, as well as studies showing it causes fewer side effects, may also help convince people who have been sceptical of other Covid-19 vaccines.
'Nuvaxovid is distinct from other Covid-19 vaccines currently in use in the UK as it uses recombinant protein-based technology which has been used for many years in the development of vaccines to prevent other illnesses, for example hepatitis B,' Sir Munir Pirmohamed, the chair of the independent Commission on Human Medicines, said of the newly-approved vaccine.
Advert
If you have a story you want to tell send it to UNILAD via [email protected]
Topics: Coronavirus, UK News, Now
